On Earth, water follows well-known physical rules: it freezes below 0 °C and boils above 100 °C. But on icy moons like Europa or Enceladus—worlds with extremely thin or even absent atmospheres—water behaves in fundamentally different and unstable ways. Under such conditions, it can boil and freeze simultaneously. While no liquid water has been observed on the surfaces of these moons, it may still intermittently emerge through a process known as effusive cryovolcanism—the eruption of water or other volatile substances from the subsurface, driven by heat or pressure deep below the surface. Yet, until now, the precise behaviour expected of water erupting into the low-pressure surface environments of icy moons remained poorly understood. A new scientific study led by Czech researchers, in collaboration with colleagues from The Open University and the University of Sheffield in the UK, offers answers. Their findings were recently published in the journal Earth and Planetary Science Letters (EPSL).
The team, led by Petr Brož from the Institute of Geophysics of the Czech Academy of Sciences, investigated how water transitions into ice under low-pressure conditions like those found on the surfaces of many bodies in the Solar System. The researchers placed several litres of water into ‘George’, a specialized low-pressure chamber at The Open University in the UK, which simulates space environments such as those on the surfaces of icy moons. The result? Under low pressure, the water began to boil and evaporate, despite the low temperatures. This, in turn, cooled the water, and ice began to form at the surface. The researchers observed that the ice that formed was not compact or solid—as we are used to on Earth—but instead was porous and fractured, and continually lifted and distorted by bubbles of water vapour forming as boiling continued underneath the ice.
“We found that the freezing process of water under very low pressure is much more complex than previously thought,” says Petr Brož, lead author of the study. “In such conditions, water rapidly boils even at low temperatures, as it is not stable under low pressure. Simultaneously, it evaporates and begins to freeze—driven by the intense cooling effect caused by the evaporation itself. The ice crust that forms is repeatedly disrupted by vapour bubbles, which lift and fracture the ice, significantly slowing down, complicating, and prolonging the freezing process.”
This phenomenon may have major implications for our understanding of cryovolcanism, an exotic process thought to have occurred on icy planetary bodies such as Europa, Enceladus, Ceres, or Pluto, during which water or other volatile substances erupted from underground onto the surface. And not just there. Similar conditions exist on Mars as well, where this study could help explain some still poorly understood surface features associated with the release of liquid water on the Red planet.
“The ice layer that forms is weak and full of holes and bubbles,” explains Frances Butcher, co-author of the study from the University of Sheffield. “If the ice was stronger, it would likely seal-off the liquid water below and prevent further boiling. But our experiments show that as the water boils, the gas that is released gets trapped under the icy crust. Pressure builds, the ice cracks, the gas escapes, and liquid water can briefly seep through the cracks onto the surface of the ice—only to be exposed again to the low-pressure environment. As soon as new fractures appear, water begins to boil again, and the entire process repeats itself.”
The researchers hope their experiments will help identify ancient signs of cryovolcanic activity not only on icy moons but across other celestial bodies in the Solar System.
“These topographic irregularities—caused by trapped vapour beneath the ice—may leave distinct signatures that could be detectable by orbiting spacecraft, for example by those equipped with radars, offering a potential new way to identify ancient cryovolcanic activity. This could provide valuable clues for planning future missions to these remote worlds—and help us better understand the still mysterious process of cryovolcanism.” adds Manish Patel from the Open University.
The research was funded by the Czech Science Foundation.
More information:
Mgr. Petr Brož, Ph.D.
Lead author
petr.broz@ig.cas.cz
Dr. Frances Butcher
Co-author
f.butcher@sheffield.ac.uk
Prof. Manish Patel
Co-author
manish.patel@open.ac.uk
Study link:
will be added later
Multimedia materials:
https://www.dropbox.com/scl/fo/a1aymyo177xov3c00z6kv/AMnYW698zqbS8haSID5zB0Y?rlkey=rytpvb05xhuourbt2xbb8g0fk&dl=0

Figure 1: Vojtěch Patočka, co-author of the study, working on an experiment using the “George” low pressure chamber at The Open University in the UK.

Movie 1: A five times sped up video showing the duel of phases during which liquid water turns into ice, but at the same time boils due to low pressure. This creates a crust of ice that is repeatedly broken up, hence, extending the time it takes for the water to boil.
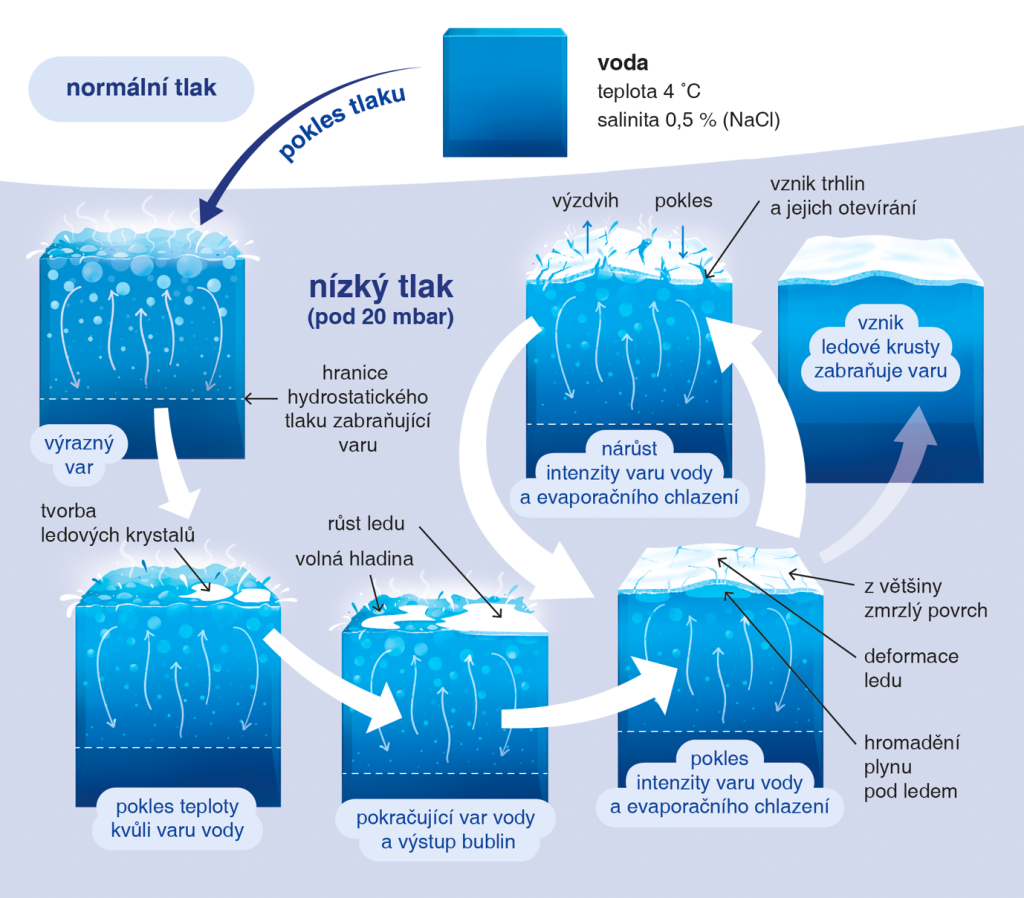
Figure 2: Schematic model showing the main phases associated with the phase transition of water under reduced atmospheric pressure.

